Home Products C13H19N5O6 Guanosine, 2′ -O-(2-methoxyethyl)- (9CI, ACI)
C13H19N5O6 Guanosine, 2′ -O-(2-methoxyethyl)- (9CI, ACI)
Shop the highest quality C13H19N5O6 Guanosine, 2′ -O-(2-methoxyethyl)- (9CI, ACI) at our factory. Get the best deals on this product for your research and production needs.
Request a Quote
PRODUCTS DETAILS
CAS Registry Number 473278-54-5 Key Physical Properties Value Condition Molecular Weight 341.32 - Boiling Point (Predicted) 715.0±70.0 °C Press: 760 Torr Density (Predicted) 1.81±0.1 g/cm3 Temp: 20 °C; Press: 760 Torr pKa (Predicted) 13.20±0.70 Most Acidic Temp: 25 °C
Canonical SMILES O=C1N=C(N)NC2=C1N=CN2C3OC(CO)C(O)C3OCCOC Isomeric SMILES O(CCOC)[C@H]1[C@@H](O[C@H](CO)[C@H]1O)N2C3=C(N=C2)C(=O)N=C(N)N3 InChI InChI= 1S/C13H19N5O6/c1-22-2-3-23-9-8(20)6(4-19)24-12(9)18-5-15-7-10(18)16-13(14)17-11(7)21/h5-6,8-9,12,19-20H,2-4H2,1H3,(H3, 14,16,17,21)/t6-,8-,9-,12-/m1/s1 InChI Key DLLBJSLIKOKFHE-WOUKDFQISA-N 1 Other Name for this Substance 2′ -O -(2-Methoxyethyl)guanosine (ACI) Properties available Optical and Scattering Thermal Property Value Condition Source Optical Rotatory Power -51 deg-mL/g-dm c: 1.4 g/100ml; Solvent: Dichloromethane; λ: 589.3 nm; Temp: 25 °C (1) CAS Optical Rotatory Power See Full Text (2) CAS
(1) Wen, Ke; Journal of Organic Chemistry, (2002), 67(22), 7887-7889, CAplus (2) Wen, Ke; Journal of Organic Chemistry, (2002), 67(22), 7887-7889, CAplus Property Value Condition Source Melting Point See Full Text (1) CAS
(1) Taj, Shabbir Ali S.; Nucleosides, Nucleotides & Nucleic Acids, (2008), 27(9), 1024-1033, CAplusSpectra available 1 H NMR 13 C NMR IR Properties available Biological Chemical Density Lipinski Structure Related Thermal Property Value Condition Source Bioconcentration Factor 1.0 pH 1; Temp: 25 °C (1) ACD Bioconcentration Factor 1.0 pH 2; Temp: 25 °C (1) ACD Bioconcentration Factor 1.0 pH 3; Temp: 25 °C (1) ACD Bioconcentration Factor 1.0 pH 4; Temp: 25 °C (1) ACD
Property Value Condition Source Bioconcentration Factor 1.0 pH 5; Temp: 25 °C (1) ACD Bioconcentration Factor 1.0 pH 6; Temp: 25 °C (1) ACD Bioconcentration Factor 1.0 pH 7; Temp: 25 °C (1) ACD Bioconcentration Factor 1.0 pH 8; Temp: 25 °C (1) ACD Bioconcentration Factor 1.0 pH 9; Temp: 25 °C (1) ACD Bioconcentration Factor 1.0 pH 10; Temp: 25 °C (1) ACD
(1) Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994-2023 ACD/Labs) Property Value Condition Source Koc 1.0 pH 1; Temp: 25 °C (1) ACD Koc 1.0 pH 2; Temp: 25 °C (1) ACD Koc 4.27 pH 3; Temp: 25 °C (1) ACD Koc 8.49 pH 4; Temp: 25 °C (1) ACD Koc 9.43 pH 5; Temp: 25 °C (1) ACD Koc 9.54 pH 6; Temp: 25 °C (1) ACD Koc 9.55 pH 7; Temp: 25 °C (1) ACD Koc 9.55 pH 8; Temp: 25 °C (1) ACD Koc 9.55 pH 9; Temp: 25 °C (1) ACD Koc 9.54 pH 10; Temp: 25 °C (1) ACD logD -2.65 pH 1; Temp: 25 °C (1) ACD logD -1.84 pH 2; Temp: 25 °C (1) ACD logD -1.08 pH 3; Temp: 25 °C (1) ACD logD -0.78 pH 4; Temp: 25 °C (1) ACD logD -0.74 pH 5; Temp: 25 °C (1) ACD logD -0.73 pH 6; Temp: 25 °C (1) ACD logD -0.73 pH 7; Temp: 25 °C (1) ACD logD -0.73 pH 8; Temp: 25 °C (1) ACD logD -0.73 pH 9; Temp: 25 °C (1) ACD logD -0.73 pH 10; Temp: 25 °C (1) ACD logP -0.730±0.568 Temp: 25 °C (1) ACD Mass Intrinsic Solubility 0.44 g/L Temp: 25 °C (1) ACD Mass Solubility 38 g/L pH 1; Temp: 25 °C (1) ACD Mass Solubility 5.5 g/L pH 2; Temp: 25 °C (1) ACD
Property Value Condition Source Mass Solubility 0.96 g/L pH 3; Temp: 25 °C (1) ACD Mass Solubility 0.48 g/L pH 4; Temp: 25 °C (1) ACD Mass Solubility 0.44 g/L pH 5; Temp: 25 °C (1) ACD Mass Solubility 0.44 g/L pH 6; Temp: 25 °C (1) ACD Mass Solubility 0.44 g/L pH 7; Temp: 25 °C (1) ACD Mass Solubility 0.44 g/L pH 8; Temp: 25 °C (1) ACD Mass Solubility 0.44 g/L pH 9; Temp: 25 °C (1) ACD Mass Solubility 0.44 g/L pH 10; Temp: 25 °C (1) ACD Mass Solubility 0.44 g/L Unbuffered Water pH 7.20; Temp: 25 °C (1) ACD Molar Intrinsic Solubility 1.3 x 10-3 mol/L Temp: 25 °C (1) ACD Molar Solubility 0.11 mol/L pH 1; Temp: 25 °C (1) ACD Molar Solubility 0.016 mol/L pH 2; Temp: 25 °C (1) ACD Molar Solubility 2.8 x 10-3 mol/L pH 3; Temp: 25 °C (1) ACD Molar Solubility 1.4 x 10-3 mol/L pH 4; Temp: 25 °C (1) ACD Molar Solubility 1.3 x 10-3 mol/L pH 5; Temp: 25 °C (1) ACD Molar Solubility 1.3 x 10-3 mol/L pH 6; Temp: 25 °C (1) ACD Molar Solubility 1.3 x 10-3 mol/L pH 7; Temp: 25 °C (1) ACD Molar Solubility 1.3 x 10-3 mol/L pH 8; Temp: 25 °C (1) ACD Molar Solubility 1.3 x 10-3 mol/L pH 9; Temp: 25 °C (1) ACD Molar Solubility 1.3 x 10-3 mol/L pH 10; Temp: 25 °C (1) ACD Molar Solubility 1.3 x 10-3 mol/L Unbuffered Water pH 7.20; Temp: 25 °C (1) ACD Molecular Weight 341.32 pKa 13.20±0.70 Most Acidic Temp: 25 °C (1) ACD pKa 3.00±0.20 Most Basic Temp: 25 °C (1) ACD Vapor Pressure 1.86 x 10-21 Torr Temp: 25 °C (1) ACD
(1) Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994-2023 ACD/Labs) Property Value Condition Source Density 1.81±0.1 g/cm3 Temp: 20 °C; Press: 760 Torr (1) ACD Molar Volume 188.5±7.0 cm3/mol Temp: 20 °C; Press: 760 Torr (1) ACD
(1) Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994-2023 ACD/Labs) Property Value Condition Source Freely Rotatable Bonds 8 (1) ACD H Acceptors 11 (1) ACD H Donors 5 (1) ACD H Donor/Acceptor Sum 16 (1) ACD logP -0.730±0.568 Temp: 25 °C (1) ACD Molecular Weight 341.32
(1) Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994-2023 ACD/Labs) Property Value Condition Source Polar Surface Area 153 A2 (1) ACD
(1) Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994-2023 ACD/Labs) Property Value Condition Source Boiling Point 715.0±70.0 °C Press: 760 Torr (1) ACD Enthalpy of Vaporization 109.72±3.0 kJ/mol Press: 760 Torr (1) ACD Flash Point 386.2±35.7 °C (1) ACD
(1) Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994-2023 ACD/Labs)Spectra available 1H NMR 13C NMR
Hot Products
C43H55N4O10P Uridine, 5′ -O- [bis(4-methoxyphenyl)phenylmethyl]-2′ -O-(2-methox yethyl)- 5-methyl-, 3′ - [2-cyanoethyl N,N-bis(1-methylethyl)phosphor amidite] (ACI)
Aminomalononitrile p-Toluenesulfonate
1,3,2-Dioxathiolane, 4-Methyl-, 2,2-dioxide, (4R)
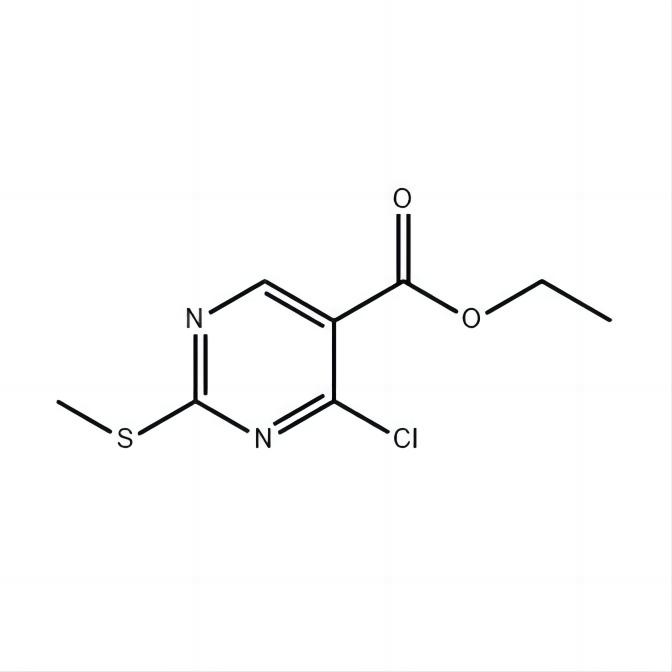
Ethyl 4-chloro-2-methylthio-5-pyrimidinecarboxylate 98%min
(R)-N-Boc-glutamic acid-1,5-dimethyl ester 98%min
(R)-4-Benzyl-2-oxazolidinone
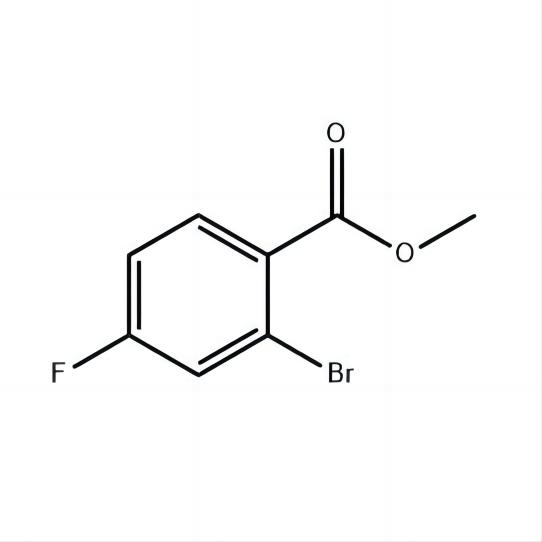
Methyl 2-bromo-4-fluorobenzoate 98%
Acrylic acid, ester series polymerization inhibitor TH-701 High Efficiency Polymerization Inhibitor
2-methoxypyrimidine 5-carboxylic acid
Acrylic acid, ester series polymerization inhibitor Hydroquinone


![C43H55N4O10P Uridine, 5′ -O- [bis(4-methoxyphenyl)phenylmethyl]-2′ -O-(2-methox yethyl)- 5-methyl-, 3′ - [2-cyanoethyl N,N-bis(1-methylethyl)phosphor amidite] (ACI)](http://kehu02.grofrom.com/www.nvchemjs.net/877e601ce519bd028ca7ac8f1bbee479.png)